Painting a Picture of Trends at Immunology 2024
June 4, 2024
by Katie Kershaw
Thousands of scientists and clinicians descended on Chicago this May with the goal of advancing the field of immunology through the exchange of ideas and information with their fellow scientists. The venue- the 2024 AAI Conference.
Part of the mission of the AAI is the study of immunology in the pursuit of improving therapies for human disease. This sentiment was echoed by Dr. Tyler Curiel, Professor of Medicine at Dartmouth during his major symposia talk, saying we “want to get beyond making this a safe world from cancer for mice”. Cancer, and immuno-oncology remain a major focus of much of the research presented at the conference. While immune checkpoint blockade and monoclonal antibody therapy remain active areas of investigation, adoptive cell therapy (ACT) was a hot topic. With six FDA approved CAR-T therapies, all for hematological malignancies, the focus of ACT work is shifting to TILs and TCRs, and overcoming the challenges associated with turning these into successful therapies. One of these challenges is the amount of time these engineered cells exist in the host post infusion, or persistence.
The Persistence of Memory (T-Cells)
Chimeric antigen receptor (CAR) T cells, T-cell receptor (TCR), and Tumor Infiltrating Lymphocyte (TIL) based therapy are the most common types of ACT under study. CAR-T cells are engineered cells where the binding portion of an antibody is fused with T-cell signaling domains. TCR therapy uses an endogenous (or minimally modified) TCR specific for a tumor specific peptide presented by MHC. TIL therapy uses tumor biopsies to isolate tumor-specific T-cells, expand them ex-vivo and reinfuse.
All of these options have their strengths and challenges, but since all three are engineered cells, they must be expanded or edited ex vivo, before being infused into the patient. After infusion, the engineered cells must remain alive, and active in order to exert their anti-tumor effects. Therapeutic success in many studies has been correlated with increased persistence of the cell product, so it follows that this would be a major area of study, not just for the development of new therapies, but for the improvement of existing ones as well.
One known challenge to persistence in solid tumors is the upregulation of FasL, which can induce apoptosis of Fas expressing lymphocytes. Dr. Kristin Anderson of UVA presented her work to overcome this. Using a TCR recognizing mesothelin in a mouse model of ovarian cancer, Anderson created an immunomodulatory fusion protein (IFP) by fusing the ectodomain of Fas with the signaling domain for 4-1BB, so that ligation of Fas + FasL will result in a positive survival signal in place of the pro-apoptotic signal. In the TCR+IFP arm, she observed increased persistence and improved survival compared to TCR alone, in a mechanism that appeared to be predominantly due to reduced cell death (indicated by increased expression of anti-apoptotic molecules Bcl2 and Bcl-xL). Furthermore, in in-vitro co-cultures of the human version of TCR + IFP with FasL positive tumor cells, T-cells not only remained viable but produced increased amounts of IL-2, another clue that there may be enhanced persistence. Read the publication here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8915280/
Another method of targeting persistence mentioned by Dr. Christian Capitini was carefully selecting the host T-cell for engineering the CAR-T. By using a T-cell that is more antigen experienced such as a EBV+ T-cell, there are hopes to increase persistence. Much study has gone into determining what attributes T-cells with increased persistence have such as memory phenotypes or increased stemness. Since these proliferation and persistence mechanisms are generally not well understood, Megan Erwin of Vanderbilt University instead focused on understanding where tumor-specific CD8 T-cells come from. Her talk described two models - the progenitor model where self-renewing populations travel from the spleen to sites of infection or tumor, and the stochastic model, where tumor-tissue specific T-cells are not reliant on spleen replenishment. In a model of liver cancer, Erwin blocked lymphoid egress with FTY720, a sphingosine 1-phosphate receptor agonist. Upon treatment, she saw no difference in the numbers of liver tumor infiltrating T-cells, providing support for the stochastic model. In a time course isolating tumor specific T-cells, stimulating in vitro and measuring phosphorylated-Erk, Ewin observed that over time, the T-cells lost their ability to phosphorylate Erk, indicating attenuation of their TCR signaling possibly related to decreased persistence.
The suppressive tumor microenvironment (TME) presents another challenge to the persistence of infused engineered cells. Oxidative stress within the tumor can negatively affect the function of various infiltrating lymphocytes including T-cells, as well as induce apoptosis. In his talk, Nathaniel Oberholtzer from the Medical University of South Carolina presented work studying the Golgi in T-cells as a marker of this stress. Using imaging cytometry, he looked at the Golgi in exhausted T-cells and saw an increase in disruption of the Golgi structure, called dispersion. This dispersion was improved by treatment with hydrogen sulfide. Using proteomic analysis and proximity ligation assays, Oberholtzer proposed a mechanism involving oxidative stress induced loss of Prdx4 localization to the Golgi in T-cells. The addition of hydrogen sulfide conserved this co-localization. Applying these findings to ACT, cells were sorted based on high and low Golgi content and used as CD19-CAR-T cells in a melanoma model. The Golgi hi subset exhibited improved survival and better tumor control, and were present at higher frequency 14 days after transfer. Characterization and restimulation of these Golgi hi T-cells revealed increases in key cytokines, stemness markers, and a decrease in exhaustion markers.
Oberholzter’s work suggests understanding mechanisms of T-cell exhaustion can elucidate new routes to improve T-cell persistence. The implication of oxidative stress as one of these mechanisms suggests a separate field of study - metabolomic exhaustion, another trend of research presented at the conference.
The Last Supper (of Lipid Metabolites)
During the President’s Symposium, Dr. Susan Kaech from the Salk Institute shared a compelling story featuring bile acids and their effect on T-cells. As opposed to tissue resident cells which develop within the tissue they live in, T-cells are introduced to an organ completely foreign to them, necessitating the need to adapt. Since tumors arise from a variety of organs, Kaech impassioned the importance of considering how the tumor tissue of origin can affect the immune response, particularly the different metabolic activities of those tissues. “Signal four” Kaech added to the traditional T-cell activation paradigm of signal one antigen, signal two co-stimulation, signal three cytokines, “is nutrients.”
“Signal four is nutrients”
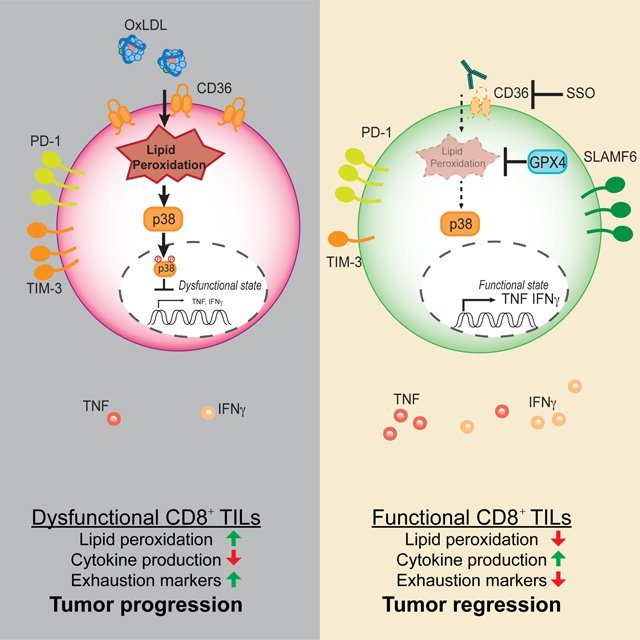
In models of colorectal cancer (MC38) and melanoma (B16), Kaech’s lab studied the metabolomics of the tumor interstitial fluid, affectionately termed “tumor juice”, to probe the metabolic exhaustion that occurs in tumors in the hopes of determining if there are metabolomic checkpoints or immunosuppressive metabolites. This exhaustion can change the nutrient availability for tumor infiltrating T-cells, which can affect their function. Compared to serum, oxidized lipids and polyunsaturated fatty acids (PUFA) including arachidonic acid were more abundant in the tumor juice. As T-cells become exhausted they upregulate EP2 and EP4 to be more responsive to PGE2, a metabolite of arachidonic acid. This can lead to suppression of function and cell death, ultimately causing reduced tumor persistence. These exhausted T-cells also upregulate CD36 which binds to oxidized lipids, inducing ferroptosis (an iron-dependent cell death).
Lipid buildup in the tumor microenvironment impacts immune cells. Xu et al. (2021) reveal that CD8+ T cells adapt by upregulating CD36, causing oxidized lipid accumulation and T cell dysfunction.
In the presented work, led by postdoctoral fellow Dr. Karthik Varanasi, Kaech described tumor reactive T-cells that were less able to efflux bile acids, which can build up in the hepatocellular carcinoma model she studies. These bile acids are toxic to T-cells, but by overexpressing the transporter SLC03A1, efflux of bile acids was rescued. Through dietary intervention, Kaech argued, primary bile acids can be regulated as a new way to treat liver cancer.
What else can these lipid metabolites do? Work from Sana Mir, a graduate student at the University of Rochester demonstrated the role of leukotriene B4 (LTB4), another lipid metabolite of arachidonic acid, in regulating CAR-T swarming. One of many challenges for ACT in solid tumors is infiltration into the tumor due to physical barriers, antigen escape, and the immunosuppressive TME. Swarming is a coordinated migration well characterized in neutrophils, but similar behavior has been observed by T-cells infiltrating tumors. LTB4, important for neutrophil swarming, was observed to be induced in cancer cells when they were treated with IFN-gamma. Mir observed CAR-T swarming in all four tumors models she tested, but this swarming was abolished when tumors were treated with an antagonist for the high affinity receptor of LTB4, BLT1, suggesting that the lipid signaling molecule LTB4 has an important role in in ACT infiltration.
Lipid signaling molecules affect other immune cells as well. Paul Bunk of Cold Spring Harbor Laboratory described the role of PPARdelta, a master regulator of fatty acid oxidation, in regulation of Treg suppressive activity within tumors in a CD8 T-cell dependent manner. On the myeloid front, Dr. Dimitry Gabrilovich of Astrazeneca detailed the conversion of neutrophils into highly immunosuppressive PMN-MDSCs by induction of ferroptosis. A targeted knockout of the enzyme involved in esterification of PUFAs (ASCL4) abrogated the immunosuppressive activity of these neutrophils. Gabrilovich stressed that for myeloid cells, functional states should be the focus instead of the myriad of populations that have been described by single-cell sequencing, advanced flow cytometry and CyTOF. “This is great biology only for the purpose of publishing papers.” he quipped. The professor continued by suggesting the best possible therapeutic success will be a pleiotropic approach targeting multiple cell types. Targeting shared metabolic messengers in tumor settings is one approach.
Outside of oncology, lipid metabolism defects were shown to mediate Th17 effects in type 2 diabetes (T2D). Naveena Ujagar of UCI described her work isolating pathogenic and non-pathogenic Th17 subsets form both mouse and human samples. Using Nanovials, Ugagar was able to capture the secreted cytokines to confirm Th17 identity at single-cell resolution, followed by sequencing to compare the differentially expressed genes of the different subsets. The human pathogenic Th17 subset displayed upregulation of fatty acid metabolism genes, hinting that it may be possible to skew this subset towards a non-pathogenic phenotype by manipulating metabolic genes.
This fine tuning of the immune system to improve therapeutic responses was a recurring theme throughout many translational talks at the AAI conference. But to be successful at this manipulation, scientists need to better understand the basic biology that governs the development of immune cells and the immune response.
The Creation of Adam(‘s Immune System)
While the star of the ACT show may be T-cells, understanding how both arms of the adaptive immune system work together in cancer should be given more attention according to Dr. Jose Conejo-Garcia of Duke University. “We teach our students both arms work in coordination and reinforce each other. This has been true in every other biological context except cancer” Dr. Conejo-Garcia stated. Work presented from his lab detailed the association of intra-tumoral plasma cells (PC) in high grade serous carcinoma with better outcome. The majority of these PCs produced IgA.
IgA is produced as a dimer and can undergo transcytosis via the IgA/IgM polymeric immunoglobulin receptor (PIGR) which is expressed on epithelial cells, including many tumor cells. Conejo-Garcia produced mutant Kras specific antibodies of both IgGA1 and IgG4 isotypes and treated a mouse model of ovarian cancer. Only the IgA antibody was able to neutralize mutant Kras within the tumor cells, underscoring the importance of understanding the biology of the humoral response in tumor therapy. “T-cells are the star of the movie of immuno-oncology, but they aren’t the only component” Conejo-Garcia concluded. Read more about this work here: https://www.cell.com/immunity/fulltext/S1074-7613(23)00418-1
“There’s a big difference between memory and constant reminder.”
The differentiation of B-cells was another prominent topic. Roy Mulpur of Emory University presented his CRIPSRgenic system to study the factors that push B-cells to their diverse subsets, memory, plasma cell, extrafollicular etc., and how different antigen types influence this. The mysterious B-cell subset of regulatory B-cells or Bregs, was discussed by Nathan Meinhardt of Medical College of Wisconsin. In the context of the autoimmune disorder multiple sclerosis, deletion of a gene necessary for B-cell maturation in a mouse model (EAE) causes failure of the mice to recover, while adoptive transfer of B-cells attenuates EAE and drives the expansion of Tregs. Determining the markers and signature of Bregs is an ongoing area of study - one Meinhardt approached by using CITE-seq to manually gate human B-cell populations based on surface protein expression. Using single modality single-cell RNA sequencing can result in what Meinhardt called “cellular ghosts” - clusters with unique genes but no biological relevance as they can not be validated at the protein level. This approach to understanding Breg protein and gene signatures in the context of disease can be used to find other rare cell subsets in humans according to Meinhardt. This work also underscores that protein does not always equal transcript.
Not long to remain out of the spotlight- understanding the development of antigen-specific CD4 Memory T-cells was the topic of Dr. Mark Jenkins’ president’s symposium talk. Memory T-cells are the survivors of a contraction phase that results in 90% cell death, yet this is the least understood aspect of the immune response according to Jenkins. While there is a robust model for CD8 T-cells, this is not true for CD4. The lab developed a peptide-MHCII tetramer system and used a mutant strain of listeria that gets cleared from mice in 3-4 days to study the memory response. The choice of infection was important since Jenkins stated it is necessary for the stimulus to be gone when studying immunological memory. “As my wife says,” the esteemed professor joked, “there’s a big difference between memory and constant reminder.”
After extensive flow cytometry and sequencing analysis, Jenkins described a model for CD4 memory in which proliferating cells rapidly differentiate, bifurcate to become Th1 and Tfh effector cells, but most cells die even after exiting the cell cycle. Rare cells differentiate, then survive. This contrasts to the established CD8 model described by survive, then differentiate, where most proliferating cells die, but the survivors exit cell cycle and become quiescent. Single-cell sequencing studies helped confirm this CD4 hypothesis, wherein early during infection there are two, in Jenkins’ words, “blobs” (clusters on UMAP) where CD4s have differentiated, but at day 21 there is one “blob” of Memory cells (the survivors). Imparting his wisdom Jenkins said, “like all of you, I’ve done a lot of single-cell RNA sequencing experiments. Haven’t learned much. Until I had a hypothesis.”
Finishing Touches
Studying immune cells at single-cell resolution was a central theme of translational research throughout the conference. Nathan Meinhardt highlighted the issue of “cellular ghosts”—unique transcript populations that lack biological relevance. To address this, he emphasized the importance of focusing on protein levels in immune subsets. Partillion’s Nanovial technology enhances the immunology toolbox by enabling scientists to capture and analyze secreted proteins from individual cells. This provides valuable insights into immune cell behavior, persistence, and functionality. By integrating Nanovials with advanced flow cytometry and single-cell RNA sequencing, researchers can overcome challenges like cellular "ghosts" and gain a better understanding of the basic biology driving therapeutic responses. Ultimately, this supports the development of more effective immunotherapies and advances immunological research.
Learn How Researchers Are Using Nanovials To Interrogate Immune Cells
Visit the Publications Page