Top Trends in Immunotherapy – Festival of Biologics 2023 Recap
October 25th, 2023
In the heart of Basel, a short walk from the Rhine River, the Festival of Biologics 2023 gathered experts and enthusiasts alike to discuss the drug development process. Over the course of three days, attendees learned about the latest developments in immunotherapy, challenges in clinical trials and the commercialization process. This recap highlights key trends, offering a glimpse into the advancements shaping the future of biologics and healthcare.
Multispecifics Are on The Rise, Is Added Complexity Friend or Foe?
Monoclonal antibodies are the most commonly used immunotherapy. Engineered to be highly targeted, they can be very effective in treating various diseases, including cancer, autoimmune disorders, and infectious diseases. However, immune evasion poses a challenge for monoclonal antibodies in therapy as pathogens or cancer cells can mutate or downregulate target antigens, evading recognition, making it difficult for antibodies to bind effectively . Additionally, the immune system can produce neutralizing antibodies against therapeutic monoclonal antibodies, diminishing their effectiveness. To address these challenges, multispecific antibodies (MsAbs) are on the rise, with one third of all new oncology antibody clinical trials started this year being for MsAbs. Generally, MsAbs target multiple antigens, bring two different biomolecules together to initiate a function, or can engage T cells to a target (BiTEs). Multispecific antibodies are also attractive as they can leverage regulatory processes typically used for monoclonal antibodies without doubling the cost.
There was much debate on the benefits and risks or MsAbs in the Keynote panel discussion, “Engineering Next Generation Therapeutics – the future of biologic development”. Louis Boon, CSO of JJP Biologics, argued that it is better to understand the mechanisms behind immune evasion or lack of efficacy for certain patients and focus on finding new biomarkers versus making more complex systems. For example, monoclonal IgG4 antibodies have been proven to be both effective and safe. In contrast, multispecific antibodies have not been studied as extensively, lacking comparable research and clinical data. The panel all agreed that easy targets are now gone so there is a need for more investigation into understanding the biological mechanisms that govern differential patient responses. The discussion rounded out with other forms of therapies such as RNA, DNA and biosimilars, and their challenges for adoption.
Manufacturing Hurdles Stand Between The Lab and The Clinic
Despite significant investments in time, money, and effort toward the development of new immunotherapies, only a small fraction of them successfully progress through clinical trials and reach the market. Once the specificity, efficacy, and immunogenicity of a therapeutic have been confirmed, establishing efficient and affordable production methods at scale remains an arduous task.
“we must pay attention to the secretome”
Addressing variability between batches of cell therapies is difficult due to the dynamic nature of living cells, making it challenging to ensure consistent outcomes. Despite batches appearing similar, the assessment of only a few cell markers for batch release makes it unclear if they are genuinely homogeneous. In the “Advances in Immunotherapy-emerging immunotherapies” panel, speaker Hans Keirstead, CEO Aivita Biomedical, highlighted that “we must pay attention to the secretome”. Selecting the appropriate secreted proteins for batch release assessment can be crucial, determining the success or failure of a therapy. There is a pressing need to better understand cell therapy variations and enhance their effectiveness by analyzing the secretome at the individual cell level. The ultimate goal is to increase the percentage of successful batches, thereby reducing overall costs.
Single B-Cell Approaches Gain Momentum
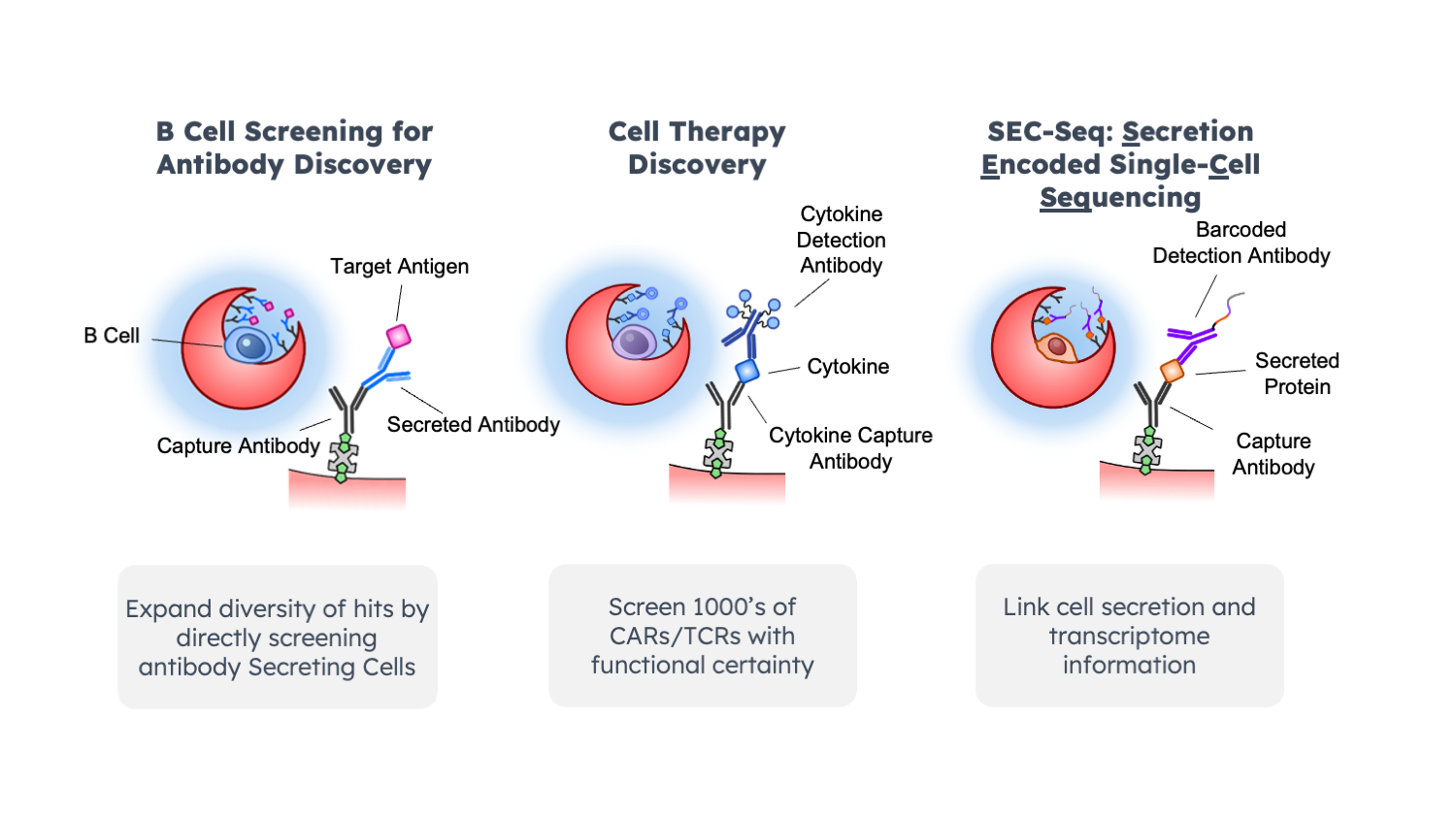
Established techniques such as phage display and hybridoma remain prevalent, but the single B-cell approach of antibody discovery is gaining momentum. For non-therapeutic purposes phage and hybridoma workflows can be cost effective while still resulting in high affinity antibodies. However, single B-cells can offer increased diversity in clones, but they come with a challenge – their delicate nature, the time-consuming workflows and the need for in-house expertise. In his talk, “Mammalian antibody display and secretion for microfluidic hit discovery”, Achim Doerner of Merck, spoke about the challenges of B cell workflows specially when it comes to building out in-house knowledge for immunizations, evaluating and establishing screening technologies, and downstream sequencing. Doerner pointed out how CROs can help facilitate some of steps like immunization but downstream steps such as sequencing can be more challenging as there are multiple unique approaches for plate based RT-PCR, leaving individuals to invest time either in creating their own methods or adapting more complex instrumentation. Although a few instruments tout all-in-one workflows, their price point puts them out of reach for many. Another challenge Doerner cited was the 12-24hr assay time typical with current instruments, "So not only scientists get tired but the cells too”. The loss of viability in these systems can lead to lower success rates. This situation frequently demands repeating the entire process, resulting in substantial losses of both time and money.
Charting the Course Ahead
Discussions at the Festival of Biologics emphasized the importance of understanding the diverse composition of the secretome. This knowledge is vital not only for analyzing our own immune system but also for evaluating the heterogeneity in therapeutics we generate. Though traditional methods allow us to do this at a bulk level, integrating new single cell approaches into existing processes might be challenging due to the substantial investment required and lengthy workflows.
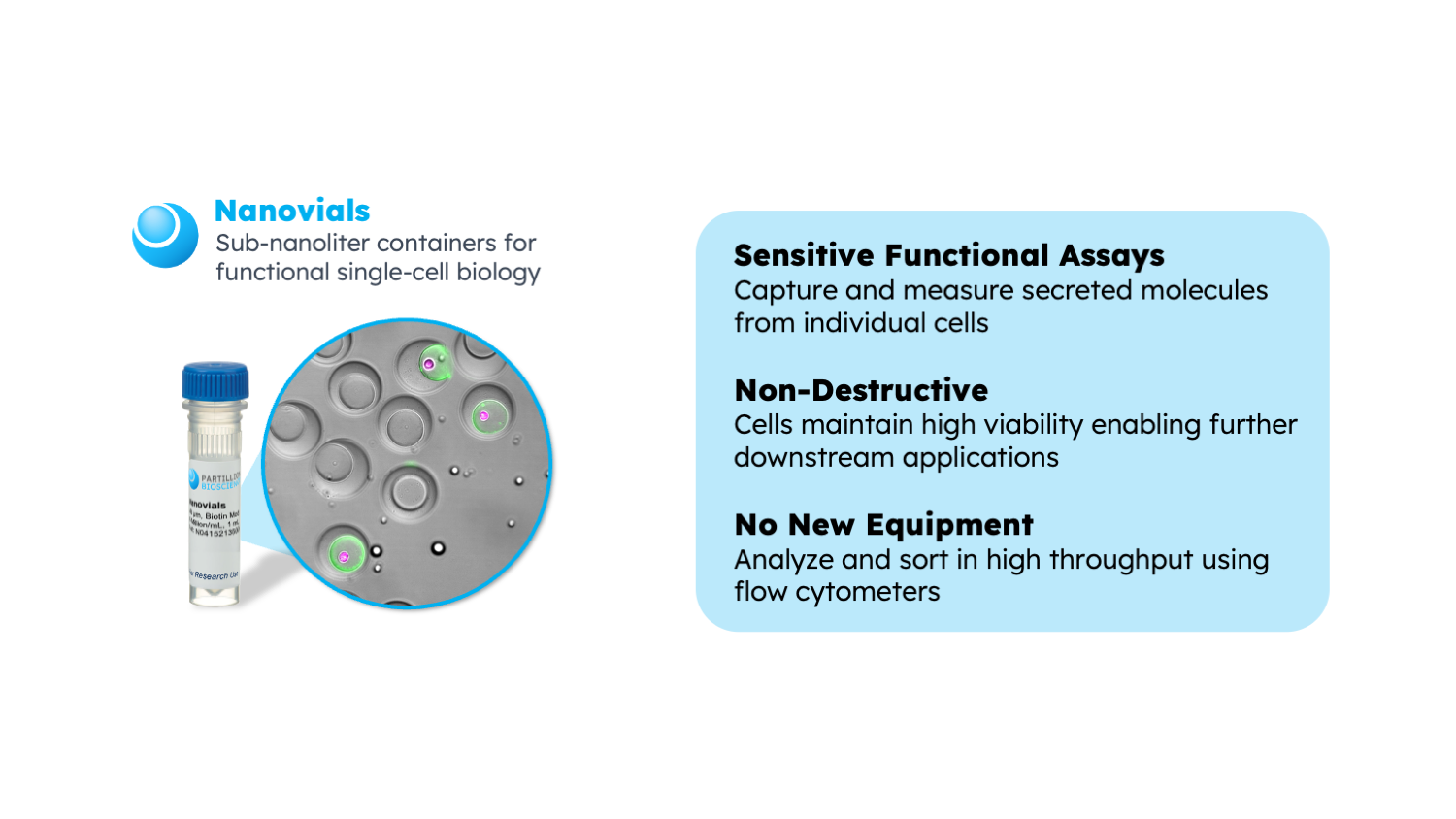
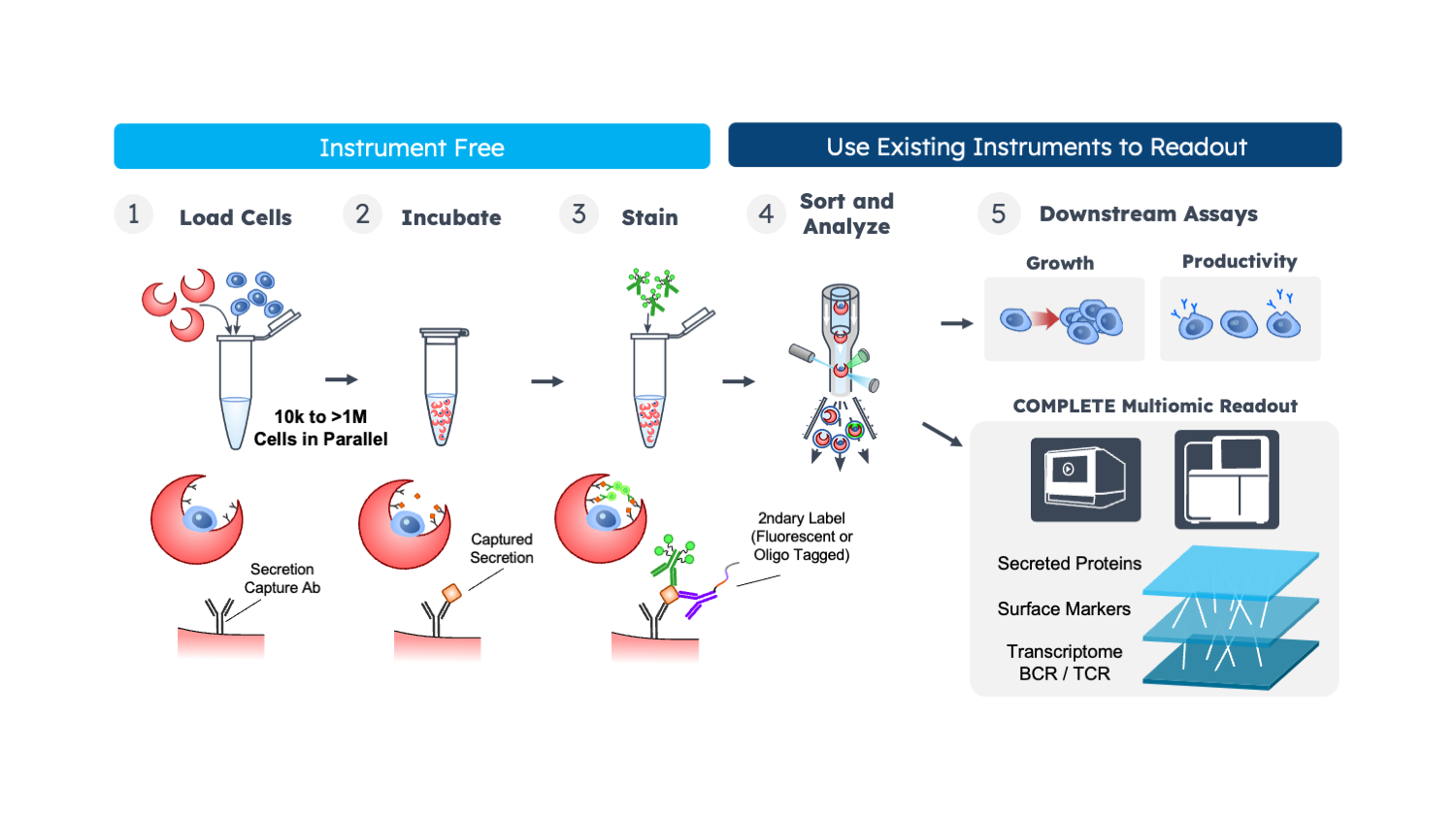
Nanovial technology simplifies the process of acquiring single cell secretome data using standard equipment. Cells are loaded into Nanovials either through capture antibodies or other affinities. Importantly, these cells remain alive, allowing them to naturally secrete proteins. These secreted proteins can then be captured and labeled with fluorescence for analysis. This method is versatile and compatible with various techniques, such as sorting through FACS instruments, sequencing, and downstream assays assessing cell growth or productivity.
The flexibility of Nanovial technology makes it easy to incorporate into existing hybridoma workflows. It reduces the hands-on time and enhances the precision of candidate screening. Additionally, B cells or CAR-T cells can be directly screened using Nanovials without destroying them. This non-destructive screening approach is crucial for subsequent cell culturing or performing additional cytotoxicity assays, allowing for a comprehensive and detailed analysis of immune cell behavior.